Deep brain stimulation (DBS) has been a pivotal intervention for neurological disorders, offering precise neuronal modulation. However, its reliance on implanted devices poses challenges. Recent advancements have introduced a photothermal wireless DBS nanosystem, termed ATB nanoparticles (ATB NPs), designed to address these limitations and provide therapeutic benefits for Parkinson’s disease (PD).
Composition and Functionality of ATB NPs
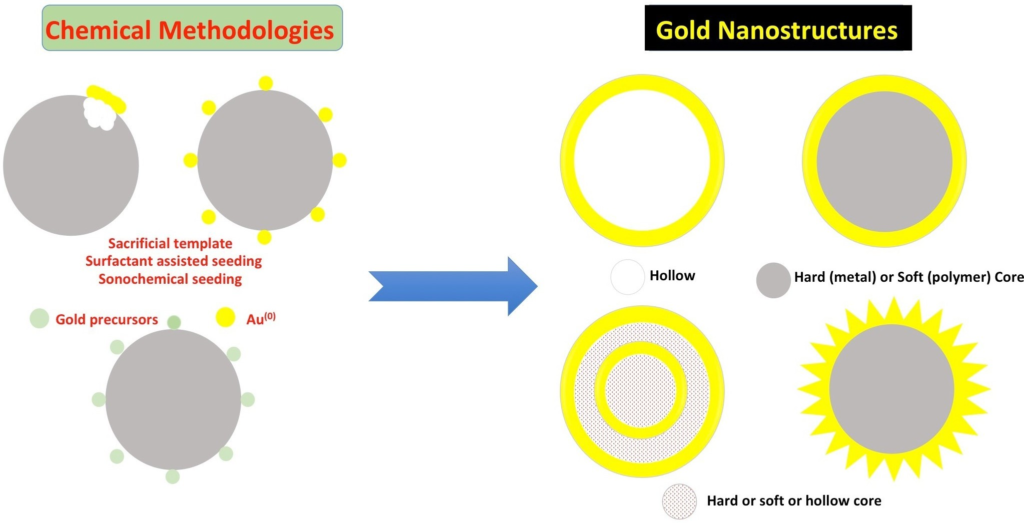
ATB NPs are engineered with three integral components:
- Gold Nanoshell (AuNSs):
Serving as the core, these nanoshells exhibit sensitivity to near-infrared (NIR) light, enabling the conversion of light to localized heat through surface plasmon resonance. - TRPV1 Antibody:
Conjugated to the AuNSs, this antibody targets the transient receptor potential vanilloid family member 1 (TRPV1) receptors, predominantly expressed on dopaminergic neurons in the substantia nigra (SN). - β-Synuclein (β-syn) Peptides:
Linked via a NIR-responsive borate ester, these peptides are released upon NIR stimulation to facilitate the disaggregation of α-synuclein fibrils.
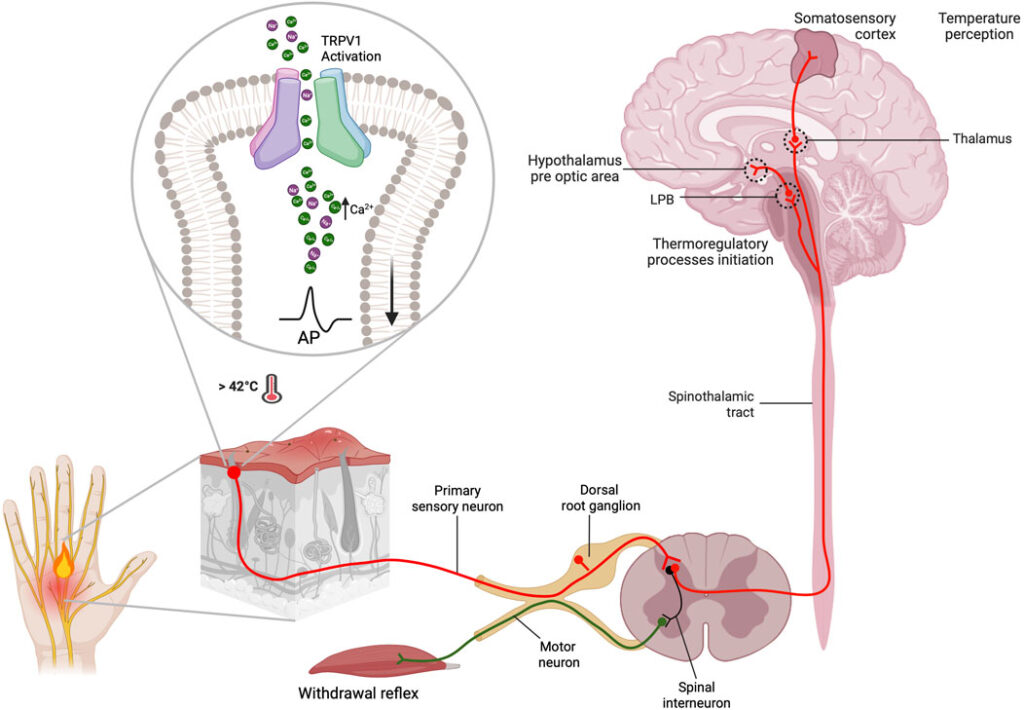
Upon stereotactic injection into the SN, ATB NPs localize to TRPV1-expressing neurons. NIR irradiation induces the AuNSs to generate mild heat, activating TRPV1 channels. This activation results in calcium ion influx, neuronal depolarization, and the initiation of action potentials. Concurrently, the released β-syn peptides promote the breakdown of α-synuclein aggregates, addressing a key pathological feature of PD.
In Vitro Efficacy
In primary dopaminergic neuron cultures, ATB NPs demonstrated targeted binding to TRPV1 receptors without inducing cytotoxicity. NIR stimulation in the presence of ATB NPs led to significant calcium influx and neuronal activation. Furthermore, in neurons treated with α-synuclein preformed fibrils (PFFs), the application of ATB NPs and NIR irradiation resulted in the disaggregation of α-synuclein aggregates and restoration of neuronal markers such as tyrosine hydroxylase (TH).
In Vivo Therapeutic Potential
In a murine model of PD induced by α-synuclein PFFs, ATB NPs were injected into the SN. The nanoparticles remained localized in the SN for at least five weeks. NIR irradiation elevated the local temperature to approximately 43°C, effectively activating TRPV1 channels. This activation was evidenced by increased expression of c-fos, an immediate early gene marker of neuronal activity. Additionally, fast-scan cyclic voltammetry revealed enhanced dopamine release in the striatum following treatment.
Histological analyses indicated that ATB NP-mediated therapy restored TH-positive neurons in the SN and striatum, reduced α-synuclein aggregates, and improved neuronal network integrity. Behavioral assessments demonstrated significant improvements in motor functions, underscoring the therapeutic potential of this approach.
Safety and Biocompatibilit

Comprehensive evaluations revealed that the heat generated by ATB NPs under NIR stimulation did not adversely affect DA neurons or other brain cells. Apoptosis assays showed no significant cell death in major brain regions. Furthermore, ATB NPs exhibited minimal translocation to peripheral organs and were associated with negligible systemic toxicity, highlighting their favorable safety profile.







